Medical Device Design Validation Plan Template . 4 tips for more efficient design verification and validation. a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company. learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. As you gather your user needs and functional requirements, consider how they will be verified and validate d so you can properly plan required activity and leave enough time to do so. guideline on process validation activities. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. if you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). Start planning v&v process early.
from templates.rjuuc.edu.np
Start planning v&v process early. As you gather your user needs and functional requirements, consider how they will be verified and validate d so you can properly plan required activity and leave enough time to do so. learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. guideline on process validation activities. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. if you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). 4 tips for more efficient design verification and validation. a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company.
Medical Device Verification And Validation Plan Template
Medical Device Design Validation Plan Template guideline on process validation activities. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. 4 tips for more efficient design verification and validation. guideline on process validation activities. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. As you gather your user needs and functional requirements, consider how they will be verified and validate d so you can properly plan required activity and leave enough time to do so. Start planning v&v process early. learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. if you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company.
From www.aplyon.com
Medical Device Process Validation Procedure ISO 13485 and FDA QSR Medical Device Design Validation Plan Template As you gather your user needs and functional requirements, consider how they will be verified and validate d so you can properly plan required activity and leave enough time to do so. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. if you develop products — medical. Medical Device Design Validation Plan Template.
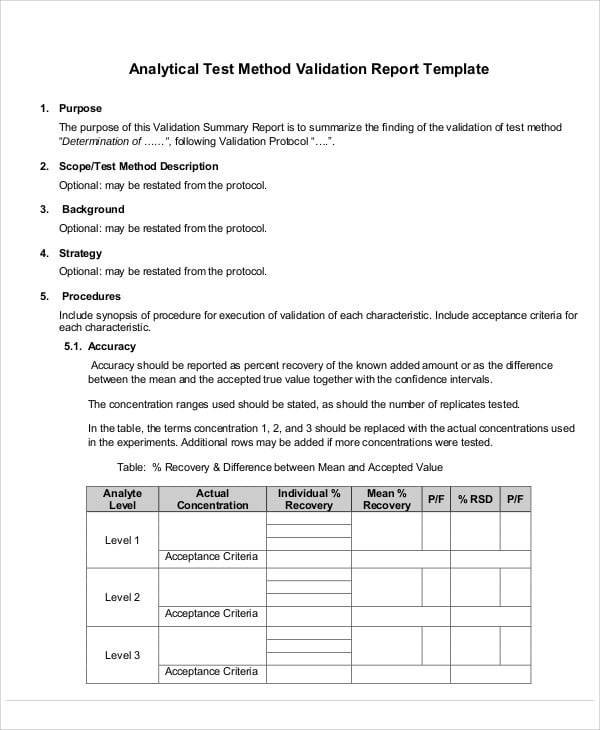
From klariti.com
Verification and Validation Plan Template (MS Word) Templates, Forms Medical Device Design Validation Plan Template a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. As you gather your user needs and functional requirements, consider how they will be verified and validate d so. Medical Device Design Validation Plan Template.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Design Validation Plan Template 4 tips for more efficient design verification and validation. if you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). International regulations for medical devices [1, 2, 3, 4] stipulate validation of. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso. Medical Device Design Validation Plan Template.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Medical Device Design Validation Plan Template International regulations for medical devices [1, 2, 3, 4] stipulate validation of. 4 tips for more efficient design verification and validation. learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. Start planning v&v process early. As you gather your user needs and functional requirements, consider. Medical Device Design Validation Plan Template.
From www.qualitymeddev.com
Design Verification vs Design Validation What are The Differences Medical Device Design Validation Plan Template learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. Start planning v&v process early. 4 tips for more efficient design verification and validation. As. Medical Device Design Validation Plan Template.
From www.aplyon.com
Design Verification and Validation Procedure Medical Device Design Validation Plan Template a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. As you gather your user needs and functional requirements, consider how they will be verified and validate d so. Medical Device Design Validation Plan Template.
From www.presentationeze.com
Validation Master Plan. Understand the importance and benefits Medical Device Design Validation Plan Template a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company. As you gather your user needs and functional requirements, consider how they will be verified and validate d so you can properly plan required activity and leave enough time to do so. learn what the validation master plan (vmp). Medical Device Design Validation Plan Template.
From prntbl.concejomunicipaldechinu.gov.co
Medical Device Verification And Validation Plan Template prntbl Medical Device Design Validation Plan Template 4 tips for more efficient design verification and validation. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. Start planning v&v process early. if you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). documentation to comply with mdr and iso 13485 (medical. Medical Device Design Validation Plan Template.
From www.perforce.com
Design Verification/Validation for Med Device Development Perforce Medical Device Design Validation Plan Template if you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). International regulations for medical devices [1, 2, 3, 4] stipulate validation of. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. 4 tips for. Medical Device Design Validation Plan Template.
From template.mapadapalavra.ba.gov.br
Medical Device Design And Development Plan Template Medical Device Design Validation Plan Template As you gather your user needs and functional requirements, consider how they will be verified and validate d so you can properly plan required activity and leave enough time to do so. 4 tips for more efficient design verification and validation. Start planning v&v process early. if you develop products — medical devices, particularly — then you’ve heard. Medical Device Design Validation Plan Template.
From quality-one.com
DVP&R Design Verification Plan and Report QualityOne Medical Device Design Validation Plan Template Start planning v&v process early. 4 tips for more efficient design verification and validation. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. guideline on process validation activities. if you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). documentation to comply. Medical Device Design Validation Plan Template.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Medical Device Design Validation Plan Template As you gather your user needs and functional requirements, consider how they will be verified and validate d so you can properly plan required activity and leave enough time to do so. guideline on process validation activities. Start planning v&v process early. 4 tips for more efficient design verification and validation. learn what the validation master plan. Medical Device Design Validation Plan Template.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Device Design Validation Plan Template a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company. Start planning v&v process early. guideline on process validation activities. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. International regulations for medical devices [1, 2, 3,. Medical Device Design Validation Plan Template.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Design Validation Plan Template a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. Start planning v&v process early. guideline. Medical Device Design Validation Plan Template.
From www.researchgate.net
Template of a validation plan. Download Scientific Diagram Medical Device Design Validation Plan Template Start planning v&v process early. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental),. Medical Device Design Validation Plan Template.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Design Validation Plan Template As you gather your user needs and functional requirements, consider how they will be verified and validate d so you can properly plan required activity and leave enough time to do so. documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso. Start planning v&v process early. a. Medical Device Design Validation Plan Template.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Design Validation Plan Template 4 tips for more efficient design verification and validation. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. guideline on process validation activities. Start planning v&v process early. learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. a free master. Medical Device Design Validation Plan Template.
From www.emmanuelbaccelli.org
Medical Device Design And Development Plan Template Medical Device Design Validation Plan Template a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company. 4 tips for more efficient design verification and validation. International regulations for medical devices [1, 2, 3, 4] stipulate validation of. learn what the validation master plan (vmp) is and how to prepare for the medical device process. Medical Device Design Validation Plan Template.